Blog
Map the latest happening in the Medical Device, BioTech, and Pharmaceutical industry
In-depth coverage of a powerful industry to assist in improving business performance
DelveInsight is a well-known Life Science market research and business consulting company noted for its collaborative market research reports as well as custom-tailored healthcare solutions.
We value strengthening our clients' businesses by providing them with sound business advice through data analysis, and assistance in accelerating their growth.

Advancing scientific innovation toward clinical development
Transforming strategic plans into smart business decisions
In-depth analysis and highlights of pipeline landscape
Excellent knowledge of regulatory systems across the world
Assist the clients in gaining a competitive edge
An in-depth analysis of assets and liabilities of entity
Improves the approach by leveraging assets and capabilities
Assists in identification and management of collaborations
Right projections of key asset performance in the future
Compares your business with others in a competitive area
Keep an eye on the pharmaceutical and healthcare market's evolving trends and news with our in-depth knowledge and assessment of the various latest pharma and healthcare subjects.
DelveInsight has gained the trust of major pharma goliaths and assisted them in achieving new heights in their business.
We provide both syndicated and customized reports, depending on the demands of our clients. Our reports provide a 360-degree panoramic picture of the market alongside latest trends.





















Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More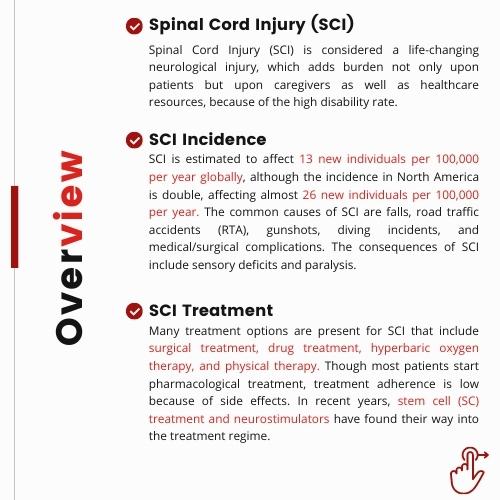
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More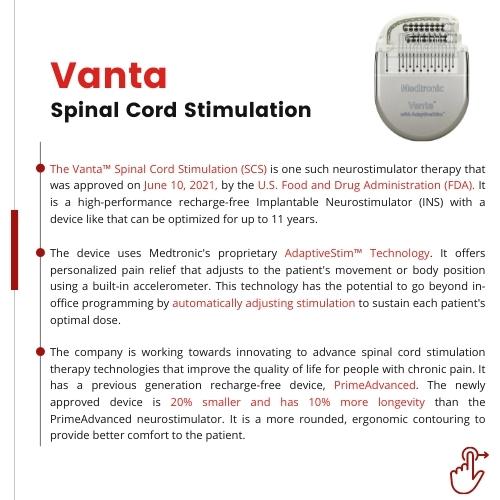
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More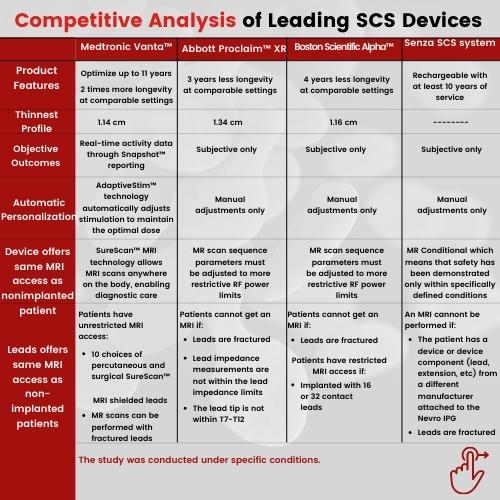
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More
Medtronic received the FDA approval for its recharge-free Implantable Neurostimulator, Vanta Spinal Cord Stimulation (SCS), with a device life optimizable up to 11 years. The company has surely set the benchmark in the recharge-free medical devices market with its device proving to be much more convenient than the other rechargeable SCS devices that demanded replacement within a few years. However, the device is expected to face stiff competition from other players in the market including Boston Scientific, Abbott, and others.
Read More